Molecular response patterns of germline STAT3 gain-of-function mutations
Maximilian Heeg
Primary immunodeficiencies provide a unique opportunity to study the human immune system, as germline mutations in patients can be directly and causally linked to their disease phenotype. Nevertheless, numerous inborn errors of immunity exhibit a significant degree of heterogeneity in the disease manifestation and penetrance. In this study, we categorized mutations in the STAT3 gene based on their molecular phenotype and associated them with the clinical penetrance and severity of the patients.
Inborn errors of immunity (IEI) provide a unique chance to study the genetic basis of autoimmune diseases. Autoimmune cytopenia, a common autoimmune manifestation, is associated with Evan’s syndrome, which combines autoimmune hemolytic anemia and thrombocytopenia in over 40% of cases. Many primary immunodeficiencies can manifest this way and are often accompanied by various autoimmune manifestations and by lymphoproliferative manifestations like splenomegaly and chronic lymphadenopathy.
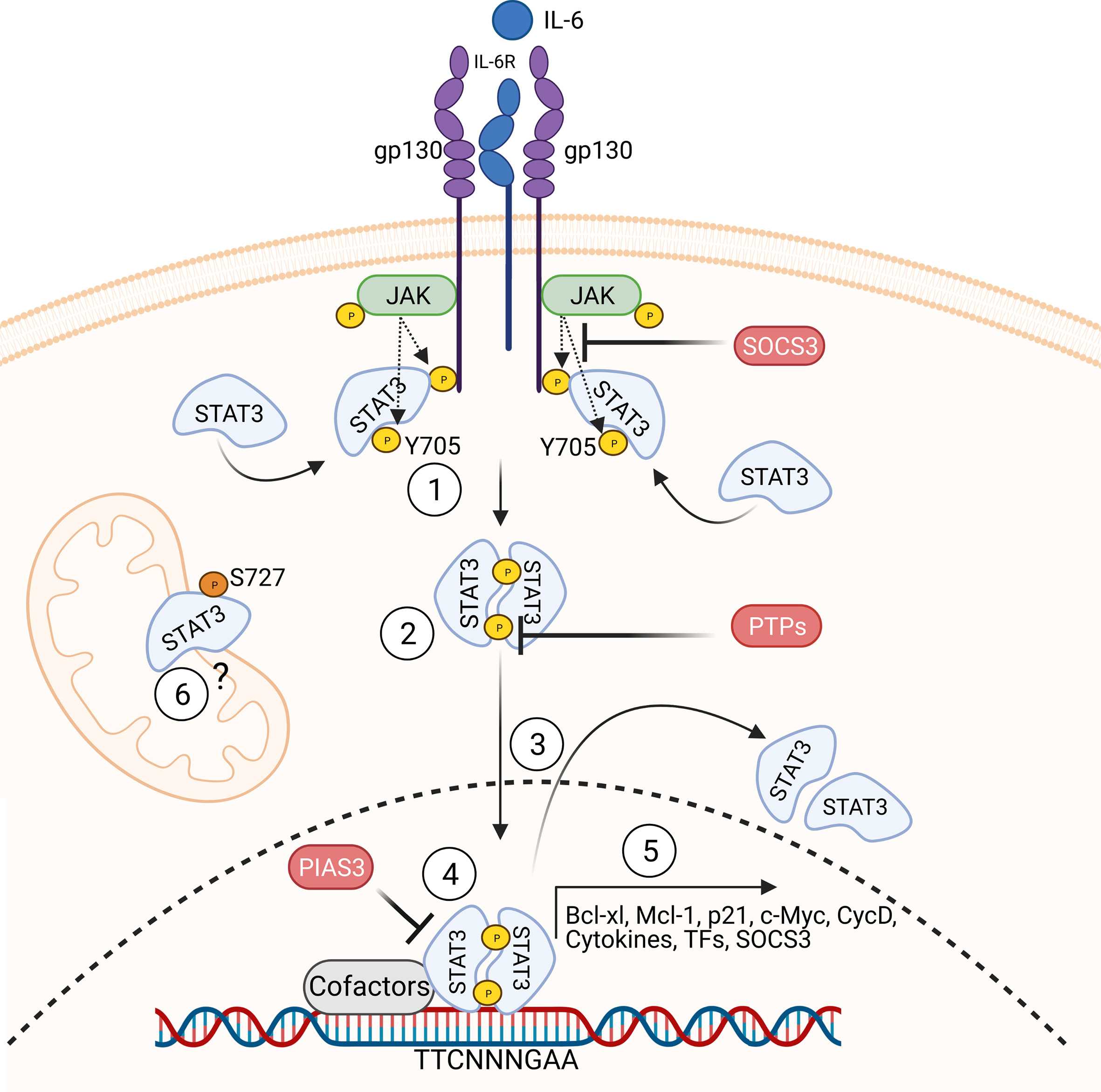
Heterozygous activating germline STAT3 mutations were initially described in patients with early-onset solid-organ autoimmunity, short stature, and eczema. Later, these reports expanded the phenotype to include autoimmune cytopenias, lymphoproliferation, and increased susceptibility to infections. STAT3 transmits signals from cytokine receptors to the nucleus, activating receptor-associated Janus kinases (JAKs) that phosphorylate tyrosine residues in the cytokine receptors, serving as STAT3 docking sites. Upon docking, STAT3 becomes phosphorylated and forms homo- or heterodimers with other phosphorylated STATs (pSTATs). These dimers translocate to the nucleus, where they bind to STAT-specific DNA binding sites and regulate the expression of target genes. STAT3 target genes regulate cellular proliferation, survival, and differentiation. Phosphatases and SOCS proteins modulate the amplitude and kinetics of STAT3 signaling.
In this study, we studied the impact of germline STAT3 mutations that have been found in patients with autoimmunity and lymphoproliferation. We find that mutations occur in all functional domains of STAT3. Most of these mutations increase transcriptional activity, as assessed by a transcriptional luciferase reporter assay. We further systematically assessed the impact of these mutations on STAT3 phosphorylation, de-phosphorylation, nuclear translocation, and DNA binding capacity and affinity and found that not all mutations have the same consequences for the different steps of the STAT3 signaling cascade, thus possibly explaining some of the clinical heterogeneity seen in these patients. To test this, we performed unsupervised clustering of results obtained in the analysis of (de-) phosphorylation, dimerization, translocation, DNA binding, and transcriptional activity, assigned the mutations to three groups defined by a distinct molecular response pattern. These mutation groups could be linked to the manifestation of autoimmune cytopenia and lymphoproliferation, confirming a mutation-determined effect on the expressivity and penetrance of STAT3 GOF-associated disease.
We envision that extending our comprehensive functional analysis to more patients with STAT3 mutations, as well as collecting more data on their association with clinical and immunological changes in larger patient populations, could pave the way for personalized therapy based on mutation groups.
Related Publications
- Clin Immunol. 20202020/distinct-molecular-response-patterns-of-activating-stat3-mutations-associate-with-penetrance-of-lymphoproliferation-and-autoimmunity
- Biomed J. 20212021/germline-stat3-gain-of-function-mutations-in-primary-immunodeficiency_-impact-on-the-cellular-and-clinical-phenotype